DOJOLVI (triheptanoin) is the first and only FDA-approved treatment for LC-FAOD.
Select a type to learn more
(carnitine palmitoyltransferase I) deficiency2,3
Cause
Mutation in the CPT1A gene; prevents long-chain fatty acids from being transported into the cells’ mitochondria for breakdown
Estimated Incidence
(carnitine-acylcarnitine translocase) deficiency2,4,5
Cause
Mutation in the SLC25A20 gene; prevents long-chain fatty acids from being transported into the cells’ mitochondria for breakdown
Estimated Incidence
(carnitine palmitoyltransferase II) deficiency2,3
Cause
Mutation in the CPT2 gene; prevents long-chain fatty acids from being transported into the cells’ mitochondria for breakdown
Estimated Incidence
(very long-chain acyl-CoA dehydrogenase) deficiency2,3
Cause
Mutation in the ACADVL gene; prevents long-chain fatty acids from being broken down via fatty acid beta-oxidation
Estimated Incidence
(trifunctional protein) deficiency2,3
Cause
Mutations in both the HADHA and HADHB genes; leads to defects in the entire TFP complex and prevents long-chain fatty acids from being broken down via fatty acid beta-oxidation
Estimated Incidence
(long-chain 3-hydroxyacyl-CoA dehydrogenase) deficiency2,3
Cause
Mutation in the HADHA gene; encodes for a subunit of TFP and prevents long-chain fatty acids from being broken down via fatty acid beta-oxidation
Estimated Incidence
LC-FAOD are a group of rare, often serious, and life-threatening autosomal recessive disorders that result from defective enzymes involved in the mitochondrial transport and catabolism of long-chain fatty acids (LCFAs). Each type of LC-FAOD is named for the specific enzyme that is affected.3,6-8
DOJOLVI IS A UNIQUE, ODD-CHAIN, MEDIUM-LENGTH FATTY ACID
DOJOLVI (triheptanoin) is a synthetic medium odd-chain (C7) triglyceride consisting of three odd-chain, 7-carbon-length fatty acids.
It is the first and only FDA-approved treatment for patients of all ages diagnosed with LC-FAOD.
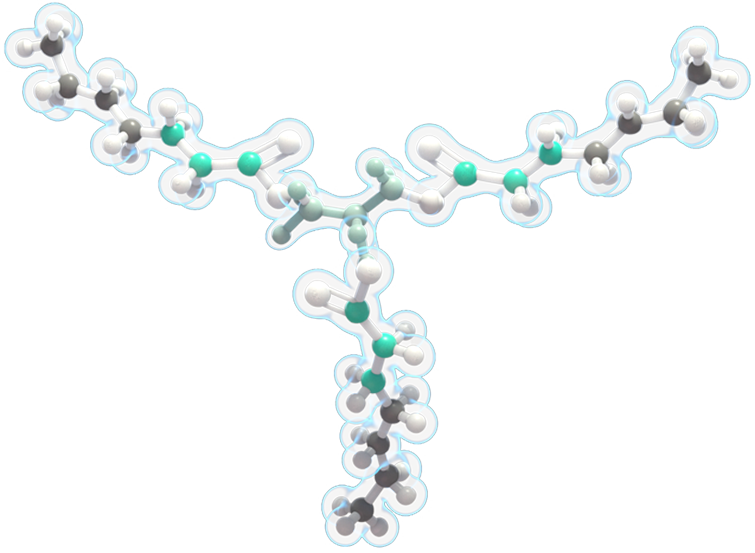
DOJOLVI (triheptanoin) is a synthetic medium odd-chain (C7) triglyceride consisting of three odd-chain, 7-carbon-length fatty acids.
It is the first and only FDA-approved treatment for patients of all ages diagnosed with LC-FAOD.
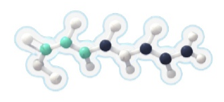
Each 7-carbon fatty acid (heptanoate) in DOJOLVI (triheptanoin) provides a source of calories and fatty acids to bypass the enzyme deficiencies in LC-FAOD for energy production and replacement.

CALCULATE DOJOLVI
Use the DOJOLVI Dosing Calculator to:
- Determine appropriate dosing for each patient
- Generate dosing reports and schedules for both you and your patients
Calculate DOJOLVI Now
Prescribe DOJOLVI
Complete the Start Form with your patient and fax it to 1–415–723–7474.
For additional support, you may also call our UltraCare Guides at 1-888-756-8657.
Access DOJOLVI
UltraCare® Patient Services provides a suite of services to help patients and caregivers:
- Gain access to DOJOLVI (triheptanoin)
- Determine eligibility for financial and patient assistance programs
- Utilize patient support program resources
- Gain access to DOJOLVI (triheptanoin)
- Determine eligibility for financial and patient assistance programs
- Utilize patient support program resources
Visit UltraCare
References:
- DOJOLVI (triheptanoin) US Prescribing Information; October 2023.
- Lindner M, Hoffmann GF, Matern D. J Inherit Metab Dis. 2010;33(5):521-526.
- Vockley J. Am J Manag Care. 2020;26(suppl 7):S147-S154.
- Pennisi EM, Garibaldi M, Antonini G. J Clin Med. 2018;7(12):E472.
- Vitoria I, Martín-Hernández E, Peña-Quintana L, et al. JIMD Rep. 2015;20:11-20.
- Knottnerus SJG, Bleeker JC, Wüst RCI, et al. Rev Endocr Metab Disord. 2018;19(1):93-106.
- Wajner M, Amaral AU. Biosci Rep. 2015;36(1):e00281.
- Wanders RJ, Ruiter JP, IJLst L, Waterham HR, Houten SM. J Inherit Metab Dis. 2010;33(5):479-494.
