DOJOLVI (triheptanoin) is the first and only FDA-approved treatment for children and adults with long-chain fatty acid oxidation disorders (LC-FAOD).

Meet DOJOLVI (triheptanoin).
WHAT IS DOJOLVI?
DOJOLVI (triheptanoin) is a prescription medicine used to treat long-chain fatty acid oxidation disorders (LC-FAOD) in children and adults.
How does DOJOLVI work?
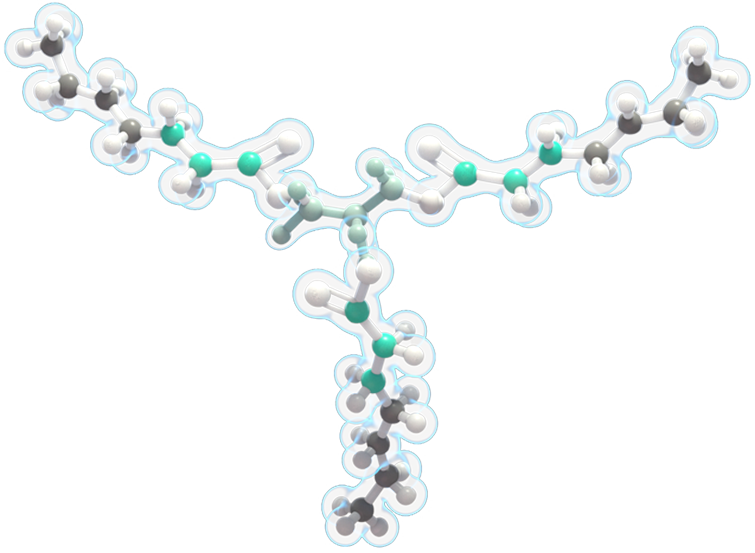
- DOJOLVI provides an alternate source of energy. It is a synthetic medium odd-chain 7-carbon triglyceride (C7).
- DOJOLVI bypasses the enzyme deficiencies that cause LC-FAOD to provide a source of calories and fatty acids that can be converted to energy.
Talk to your healthcare team to learn more about DOJOLVI.
WHAT IS DOJOLVI?
LC-FAOD defined.
WHAT ARE LC-FAOD?
Long-chain fatty acid oxidation disorders (LC-FAOD) are rare inherited disorders that prevent the body from breaking down long-chain fatty acids into energy during metabolism.
People living with LC-FAOD have difficulty producing enough energy because of their body’s inability to use long-chain fatty acids as an energy source during times of:
- Fasting
- Illness
- Prolonged exercise
Each type of LC-FAOD is named for the specific enzyme that is affected.
Select a type to learn more
(carnitine palmitoyltransferase I) deficiency
Cause
Mutation in the CPT1A gene; prevents long-chain fatty acids from being transported into the cells’ mitochondria for breakdown
Estimated Incidence
Manifestations
Key signs and symptoms manifest from birth to 18 months
(carnitine-acylcarnitine translocase) deficiency
Cause
Mutation in the SLC25A20 gene; prevents long-chain fatty acids from being transported into the cells’ mitochondria for breakdown
Estimated Incidence
Manifestations
Key signs and symptoms can present in the neonatal/infant stage of life, while late onset has been reported with less serious symptoms
(carnitine palmitoyltransferase II) deficiency
Cause
Mutation in the CPT2 gene; prevents long-chain fatty acids from being transported into the cells’ mitochondria for breakdown
Estimated Incidence
Manifestations
Key signs and symptoms are distinct in the neonatal/infant stage of life from those in adolescence/young adulthood
(very long-chain acyl-CoA dehydrogenase) deficiency
Cause
Mutation in the ACADVL gene; prevents long-chain fatty acids from being broken down via fatty acid beta-oxidation
Estimated Incidence
Manifestations
Some key signs and symptoms are present throughout life, while some that are present during early childhood are distinct from those that are present during adolescence/adulthood
(trifunctional protein) deficiency
Cause
Mutations in both the HADHA and HADHB genes, which leads to defects in the entire TFP complex; prevents long-chain fatty acids from being broken down via fatty acid beta-oxidation
Estimated Incidence
Manifestations
Some key signs and symptoms are present throughout life; those that are present in early childhood are similar to those presenting in LCHAD deficiency but are often more serious
(long-chain 3-hydroxy-acyl-CoA dehydrogenase) deficiency
Cause
Mutation in the HADHA gene, which encodes for a subunit of TFP; prevents long-chain fatty acids from being broken down via fatty acid beta-oxidation
Estimated Incidence
Manifestations
Some key signs and symptoms are present throughout life, while some that are present during early childhood are distinct from those that are present during adolescence/adulthood
WORKING WITH YOUR HEALTHCARE TEAM
All patients treated with DOJOLVI (triheptanoin) should be under the care of clinical specialists knowledgeable in appropriate disease-related dietary management based upon current nutritional recommendations.
These specialists, which may include metabolic geneticists, dietitians, and nurse practitioners, will work together as your healthcare team and:
- May start you on a low dose of DOJOLVI and slowly increase your dose to help avoid side effects
- Ensure you are receiving frequent monitoring and nutritional counseling appropriate to your specific needs when taking DOJOLVI
If you are taking another medium-chain triglyceride (MCT) product, stop taking the MCT before starting DOJOLVI.
Learn more about taking DOJOLVI

“There are going to be challenges, but take it one step at a time. Make sure you’re celebrating the good days and the milestones.”
Discover Patient Stories
Get Started.
STARTING DOJOLVI
UltraCare® Patient Services is here to help you:
- Gain access to DOJOLVI (triheptanoin)
- Understand your insurance coverage
- Determine your eligibility for financial and patient assistance programs
- Utilize patient support program resources
Visit UltraCare
A completed Start Form is required for enrollment in UltraCare Patient Services. Talk to your healthcare team.
TAKING DOJOLVI
Download this step-by-step guide to discuss with your healthcare team how to store, administer, and keep track of DOJOLVI (triheptanoin) doses.
Download the Guide